Dry Heat Sterlization
Steam sterilization uses pressurized steam at 121-132° C (250-270° F) for 30 or 40 minutes. This type of heat kills all microbial cells including spores, which are normally heat resistant. In order to accomplish the same effect with dry heat in an oven, the temperature needs to be increased to 160-170° C (320-338° F) for periods of 2 to 4 hours. 5
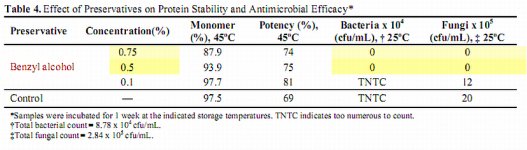
A table of most studies conducted up to 1961 indicates that they are in enough of an agreement to support the above statements from the Berkley Lab Manual indicating that 170° C (338° F) for at least 2 hours is sufficient to sterilize objects.6 (See table below)
Using more current references such as the Dental Technician Manual, Volume 1 form the U.S. Navy we find that for sterilization of metal instruments "a typical dry heat cycle is 90 minutes at 320-345°F, plus the time required to preheat the chamber." A current microbiology text book states that "the standard settings for a hot air oven sterilization are the preheat time plus 1.5 to 2 hours at 160 °C (320 °F)."7
Based on the conformity in scientific research and medical statements we should feel comfortable relying on the following information from EngenderHealth (a leading international health organization working in third world countries).
* Sterilizing by dry heat (i.e. oven). From: EngenderHealth
The temps and times to sterilize:
Place instruments and other items in the oven, and heat to the designated temperature. The oven must have a thermometer or temperature gauge to make sure the designated temperature is reached.
Use the list here to determine the appropriate amount of time to sterilize instruments and other items for different temperatures. (do not begin timing until the oven reaches the desired temperature, and do not open the oven door or add or remove any items). The times shown here represent the amount of time that items must be kept at the desired temperature to ensure that sterilization is achieved. Keep in mind that the total cycle time--including heating the oven to the correct temperature, sterilization, and cooling--is usually twice as long as the time noted here.
Temperature
170 degrees C (340 degrees F) - 1 hour
160 degrees C (320 degrees F) - 2 hours
150 degrees C (300 degrees F) - 2.5 hours
140 degrees C (285 degrees F) - 3 hour
I suggest that as long as your glassware is capable, that the highest temperature (170 degrees C (340 degrees F) ) be used for twice the stated time... making the safe time 2 hours.
REFERENCES
1 - Development of a Multidose Formulation for a Humanized Monoclonal Antibody Using Experimental Design Techniques, Supriya Gupta, AAPS PharmSci 2003; 5 (2) Article 8
2 - A Contribution to our Knowledge of Disinfectant Action. III. Unsaturated Compounds as Germicides, H. D. Cheeseworth, E. A. Cooper, J. Phys. Chem., 1929, 33 (5), pp 720–728
3 - The veterinary formulary By Yolande M. Bishop, British Veterinary Association Pharmaceutical Press; 6 edition (October 30, 2004) page 359
4 - Disinfection, sterilization, and preservation By Seymour Stanton Block, Lippincott Williams & Wilkins; Fifth Edition edition (December 15, 2000)
5 - Biosafety Manual, Berkley National Laboratory
http://www.lbl.gov/ehs/biosafety/Bio...lization.shtml
6 - Sterilization by dry heat, E. M. Darmady, K. E. A. Hughes, J. D. Jones, D. Prince, and Winifred Tuke, J Clin Pathol 1961; 14: 38-44
7 - Textbook of Microbiology by Ananthanarayan and Panikar, Orient Longman; 7Rev Ed edition (December 6, 2006)